Can Autophagy Help Repair Back Discs
Abstract
There is one circadian clock in the primal nervous system and another in the peripheral organs, and the latter is driven by an autoregulatory molecular clock composed of several cadre clock genes. The height, h2o content, osmotic pressure and mechanical characteristics of intervertebral discs (IVDs) accept been demonstrated to showroom a cyclic rhythm (CR). Recently, a molecular clock has been shown to be in IVDs, abolitionism of which can atomic number 82 to stress in nucleus pulposus cells (NPCs), contributing to intervertebral disc degeneration (IDD). Autophagy is a key cellular process in eukaryotes and is essential for individual cells or organs to respond and adapt to irresolute environments; it has as well been demonstrated to occur in homo NPCs. Increasing prove supports the hypothesis that autophagy is associated with CR. Thus, we review the connection between CR and autophagy and the roles of these mechanisms in IDD.
Introduction
Approximately 2/3 of adults suffer from lower dorsum hurting (LBP), for which age-related degenerative processes in intervertebral discs (IVDs) and intervertebral disc herniation are the most common reasonsone. Each IVD is composed of a cartilage end-plate (CEP), a nucleus pulposus (NP) and an annulus fibrosus (AF)ii. All three of these components are involved in intervertebral disc degeneration (IDD)3,4. It has long been known that diurnal shifts in mechanical loading atomic number 82 to osmotic pressure changes, and these 2 types of alterations together with other microenvironmental factors stimulate nucleus pulposus cells (NPCs), contributing to the stress of NPCsv,6,7. Recently, an autoregulating circadian rhythm (CR) was identified in IVDs, the abolition of which led to IDDviii. The abolitionism of CR is likewise observed in osteoarthritis (OA) cartilage, and the degeneration of cartilage can exist induced by BMAL1 or REV-ERBα knockdown with loss of TGF-β signalingnine.
Circadian clocks are time-measuring devices that are present in most light-sensitive organisms. The central nervous system interacts with surrounding tissues with a 24-h oscillation period, decision-making nutrient metabolism, energy balance, redox status and organismal behavior and maintaining homeostasis through complicated pathways10. Autophagy is responsible for a significant connection betwixt CR and homeostasis, as volition be discussed below.
Autophagy (macroautophagy) is a fundamental cellular process in eukaryotes that is essential for individual cells or organs to reply and adapt to irresolute environments in which cells digest themselves to generate life-supporting substances11. Autophagy plays prominent roles in determining the life spans of many model organisms and in pathological processes in many organs11. Additionally, autophagy maintains the homeostasis, inhibits the apoptosis and prevents the senescence of NPCs12.
Recently, evidence of an interaction betwixt CR and autophagy has emerged. Many genes involved in dissimilar steps of the autophagy pathway, such as Atg14, UNC51-like kinase 1 (Ulk1), GABA(A) receptor-associated protein-like 1 (Gabarapl1), microtubule-associated protein i light chain 3 B (LC3B), and BCL2/adenovirus E1B-interacting protein 3 (Bnip3), have been found to be associated with CR13. In this review, we discuss how autophagy is connected to IDD and CR and what nosotros can practice as a next step to clarify the mechanisms past which CR and autophagy are involved in IDD by summarizing evidence from both in vivo and in vitro studies.
Overview of the molecular circadian clock
There are two kinds of circadian clocks: one in the fundamental nervous organization and another in surrounding tissues. The onetime is controlled by suprachiasmatic nucleus neurons following light stimulation, and the latter is controlled in every cell by the expression of clock genes14. The cell-autonomous molecular clock in mammals is generated past 2 interlocking transcription/translation feedback loops (TTFLs) that function together to produce robust 24 h rhythms of gene expression15. The cadre TTFL is driven by iv integral clock proteins, including two activators (circadian locomotor output cycles kaput (CLOCK) and brain and muscle ARNT-like protein-i (BMAL1, also known as ARNTL)) and two repressors (Period (Per) and cryptochrome (Weep)) too equally past kinases and phosphatases that regulate the phosphorylation (P) and thereby the localization and stability of these integral clock proteins (kinases: CKIα, CKIδ, and CKIɛ; phosphatases PP1, PP5)15. The cardinal principle of the molecular clock in mammals relies on a transcriptional activator inducing the transcription of a repressor, which results in the aggregating of the latter over time until information technology reaches a sufficient level to repress its ain activation16. The bones helix-loop-helix PER-ARNT-SIM (bHLH-PAS) proteins CLOCK and BMAL1 are the primary transcriptional activators within the circadian clock mechanism of mammals15. The CLOCK and BMAL1 transcription factors act as heterodimers to actuate the Per and Weep gene families through interaction with E-box regulatory sequences16. Every bit a result, the Per and Cry protein products class heterotypic complexes that accrue over fourth dimension in the cytoplasmxvi. The levels of Per/Cry complexes increment until they accomplish a threshold, marking the commencement of the repressive phase, in which the Per/Cry complexes translocate back to the nucleus and repress CLOCK/BMAL1 transactivation15. REV-ERBα/β and retinoic acrid-related orphan receptor α (RORα) are major regulators of cyclic transcription within the positive limb of the mammalian circadian oscillator, although their roles are nonessential15. Posttranslational regulation mechanisms, such every bit phosphorylation, acetylation, methylation, sumoylation, and glycosylation, so attune protein turnover, intracellular localization, and DNA-bounden analogousness16. CR then regulates NAD+ biosynthesis, amino acrid and carbohydrate metabolic pathways, and nucleotide biosynthesis, thus contributing to glucose homeostasis, energy homeostasis, hematopoietic prison cell homeostasis, epidermal stem cell homeostasis, and many other vital activities17,eighteen.
The cyclic rhythm in intervertebral discs
Many characteristics of the relationship between IVDs and CR have long been known. Body peak changes during the day19. Over 50% of the height loss in a twenty-four hour period occurs within the showtime hour of rising, and fourscore% occurs within 3 h of rising; the rate of creep decelerates throughout the residual of the waking day19. I of the reasons for these changes is pressure-dependent fluid shifting in the IVDs20. When an IVD is subjected to a diurnal bike involving 16 h of loading followed by an 8-h recovery period, the osmotic pressure level of the nucleus becomes much higher than that of the annulus, which is why fluid is fatigued back into the disc afterwards the end of loadingv. A subtract in osmotic pressure in the key nucleus region through degeneration leads to an inability to draw fluid dorsum into the discfive. The range of lumbar flexion is increased during the twenty-four hours compared to that during the dark and increases later creep, suggesting that frontwards bending movements subject the lumbar spine to college bending stresses in the early forenoon than afterwards in the day; this increase is ~300% for the discs and fourscore% for the ligaments of the neural curvation21. Discs announced to be very sensitive to their prevailing osmotic environment (at least in vitro), which has a powerful effect on matrix synthesis and, hence, ultimately on the structure and limerick of the discs during each diurnal cycle6. Penetration through the end-plate increases with diurnal loading22.
The CR of IVDs is dampened with crumbling in mice and can exist abolished by treatment with interleukin (IL)-1βeight. The genetic disruption of the mouse IVD molecular clock, specifically through the disruption of BMAL1, predisposes mice to IDD8. This finding suggests that the disruption of CR may exist a risk factor for degenerative IVD affliction and LBP (Fig. 1)8. Some upstream factors and downstream pathways that interact with CR in IVDs accept too been found. Passive cigarette smoking changes the CR of clock genes in rat IVDs, with nigh genes showing a phase shift of −half-dozen to −9 h and some clock-related genes showing abolished oscillation in the NP7. BMAL1 and RORα regulate hypoxia-inducible factor (HIF)-one activeness in NPCs and play important roles in the overall adaptation of NPCs to their hypoxic niche; the dysregulation of these proteins affects normal tissue homeostasis and function23. HIFs coordinate cellular adaptations to low-oxygen stress past regulating transcriptional programs in erythropoiesis, angiogenesis, and metabolism24.
Overview of autophagy
The canonical procedure of autophagosome formation, which is evolutionarily conserved, involves the stages of initiation, nucleation, elongation and closure, recycling and degradation25. Information technology is normally promoted by the direct activation of Ulk1 through Ulk1 phosphorylation by adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK)26. The mammalian target of rapamycin kinase circuitous i (mTORC1) is incorporated into the Ulk1–Atg13–FAK family kinase-interacting protein of 200 kDa (FIP200) circuitous, and mammalian target of rapamycin (mTOR) kinase phosphorylates Ulk1 Ser 757, disrupting the interaction between Ulk1 and AMPK and suppressing autophagy directly26,27. AMPK also phosphorylates Raptor to suppress the inhibitory effect of mTORC1 on the Ulk1 autophagic complex28.
When autophagy is induced, Ulk1 phosphorylates AMBRA1, releases the Beclin1 complex and produces phosphatidylinositol 3-phosphate (PtdIns3P or PI3P) from dynein29,xxx,31. PI3P is recognized by WD-repeat protein Interacting with Phosphoinositides (WIPI) proteins in the endoplasmic reticulum and is rapidly recruited to the site of autophagosome germination29. Beclin1 forms a complex with ER-associated Bcl-2 nether nutrient-rich conditions and is released upon the phosphorylation of Bcl-2 by c-Jun Northward-terminal kinase 1 (JNK 1)32.
Atg8 (in yeast)/microtubule-associated poly peptide 1 light chain iii (LC3) is cleaved at its C terminus by ATG4 to generate cytosolic LC3-I with a C-terminal glycine residue, which is conjugated to phosphatidylethanolamine (PE) in a reaction that requires ATG7 and the E2-like enzyme ATG333. The LC3-binding protein p62 is a specific substrate for autophagy, and the Atg12–Atg5–Atg16L1 complex is also required for the formation of the covalent bond between LC3 and PE32. The lipidated class of LC3 (LC3-2) is attached to both faces of the phagophore membrane but is ultimately removed from the autophagosome outer membrane; this removal is followed by the fusion of the autophagosome with a belatedly endosome/lysosome33. Autophagy occurs at low basal levels in most all cells to perform homeostatic functions such as protein and organelle turnover34. Autophagy is upregulated under weather condition of starvation, growth gene withdrawal, loftier bioenergetic demands, oxidative stress, infection, or protein amass accumulation34.
Autophagy in intervertebral discs
The end-plate chondrocytes (EPCs) of cervical spondylosis patients show decreased autophagy compared with those of fracture and dislocation patients35. During the aging procedure, the expression of the autophagy-related genes LC3 and Beclin-1 significantly decreases with the decreasing activity of EPCs36. In dissimilarity, autophagy has been demonstrated to occur and even to increase during normal aging in NPCs37. Autophagy has also been shown to be upregulated in AF cells (AFCs) in IDD patients38. The current findings regarding the conditions influencing autophagy in IVDs and their associated pathways and effects are summarized in the Supplementary information (Fig. 2).
Amidst these factors, tumor necrosis gene-α (TNF-α) and IL-1β regulate autophagy39,twoscore. Nevertheless, some studies accept shown that neither TNF-α nor IL-1β can regulate autophagy in rat NPCs and that IL-1β alone cannot induce autophagy in AFCs41,42. In rats, IL-1β does non induce autophagy in AFCs by itself simply augments the autophagy induced by serum deprivation; such autophagy may contribute to delays in apoptosis and IDD42. Hyperosmotic stress may activate the autophagy of NPCs via the Ca2+-dependent AMPK/mTOR pathway43. Still, this is nevertheless a controversial theory44. Specifically, mechanical loading is one of the factors that causes IDD45.
Although the mTOR pathway is involved in many of the atmospheric condition listed in the Supplementary data, the pharmacological inhibition of only mTORC1, and not mTORC2, protects against human disc apoptosis, senescence, and extracellular matrix catabolism through Akt and autophagy induction46.
The circadian rhythm of autophagy
The presence of autophagic vacuoles and the atrophy of the liver was found to follow a diurnal pattern in meal-fed rats in comparing with nutrient-deprived controls using electron microscopy47. This was the showtime prove of a link between autophagy and circadian regulation, which was established in the early 1970s47. Additional evidence of autophagy regulation by CR in the kidney, heart, liver, encephalon, and retina has been found via electron microscopy, autophagic flux measurements and fluorescence measurements, not only in mammals but also in Drosophila and zebrafish13,48,49,50,51,52,53,54,55,56,57,58,59.
The autophagic rhythm varies from tissue to tissue. In the convoluted tubules of the kidney, analyses of the number of autophagic vacuoles per area unit and the full amounts of segregated fabric have shown that the minimum values occur during the night, while the maximum values occur during the day58. The volume and numeric density of autophagic vacuoles in the middle tiptop during the late-light phase and afterward decline toward the early dark period53. Autophagy flux has been found to reach its top in the afternoon, quickly decrease at night and rise over again throughout the light stage in the mouse liverxiii. The daily rhythms of liver autophagy might not directly ascend from the molecular cyclic oscillator only may instead be indirectly coupled to the molecular clock via feeding or other normally circadian-gated behaviors56. This show suggests that autophagy can be adamant by the internal surround of a specific organ regardless of the regulation of the primal nervous system.
The cyclic rhythm regulates autophagy
Integration betwixt CR and behavior or the environment is vital for normal physiological processes. The feeding/fasting rhythm has been establish to regulate autophagy in muscle, adipose and liver tissue and to play a protective role in metabolism and life spanlx. Intermittent fasting (IF) has been shown to restore autophagic office, thereby preserving organelle quality61. The autophagy-associated protein LC3-Ii has been shown to showroom a CR of its protein expression in the hippocampus, which is dampened by sleep fragmentation (SF).51 Yet, recovery sleep did not return LC3 expression to the basal oscillating level51. The basal levels of autophagy within the outer retina change in a dynamic fashion during the course of the day and night and are regulated by the circadian calorie-free input49,52,62. In the neural retina, cyclic illumination is i of the factors that activates autophagy63.
Recently, it has been demonstrated that autophagy levels are controlled past several clock genes, specially Period2 (Per2) and BMAL1. Per2 in the liver functions as a scaffold protein to tether tuberous sclerosis complex one (TSC1), Raptor, and mTOR together to specifically suppress the activity of the mTORC1 complex64. Knockdown of Per2 downregulates autophagy levels while leaving core clock oscillations largely intact65. Knockdown of Per2 also reduces cellular levels of the Ulk1 poly peptide without affecting Ulk1 mRNA levels, consistent with the rhythmic Ulk1 protein levels and nonrhythmic Ulk1 mRNA levels in these cells65. The transient overexpression of Per2 results in the downregulation of the PI3K Class 1/Akt pathway in vitro and increases autophagic flux65,66. Tor, ATG5 and ATG7 showroom rhythmic expression in the brains of wild-type flies under day/dark conditions (LD, 12:12), which is abolished in Per1 clock mutants59. C/EBPβ is a transcription factor that is regulated past CR and induces the expression of autophagy genes such as Ulk1, Gabarapl1, LC3B, and Bnip3 and the degradation of proteins13. However, its expression and the expression of the autophagy-related genes LC3B, GABA(A) receptor-associated poly peptide a (Gabarapa), ATPase H+-transporting lysosomal V1 subunit D (Atp6v1d), ATG4a, ATG4d, Beclin1, Bnip3, Ulk1a, and Ulk1b are upregulated without rhythmicity in Per1b mutant fish50. These findings advise the indispensable role of Per in maintaining the rhythmicity of autophagy.
In the heart, BMAL1 protects cardiomyocytes under hyperglycemic conditions by inducing autophagy through mTORC1 signaling downregulation67. BMAL1−/− mice exhibit increased LC3A and decreased p62 levels in muscle, whereas Beclin-i levels are unchanged68. AMPK activation has been indicated to be involved in the regulation of BMAL1 in autophagy68. Cardiomyocyte-specific BMAL1 knockout (CBK) and cardiomyocyte-specific Clock mutant (CCM) mice exhibit hyperactivation of the PI3K Class 1/Akt/mTOR signaling axis, which likely contributes to attenuation of autophagy and the augmentation of protein synthesis69. Thus, the expression of BMAL1 may exist associated with the activation of autophagy.
In vitro, REV-ERB agonism blocks autophagy and induces apoptosis70. In vivo, REV-ERB agonism has been institute to downregulate the mRNA and protein levels of Ulk3, Ulk1, Beclin1, and ATG7 and to significantly improve survival in two glioblastoma models70. In zebrafish, REV-ERBα has been found to bind straight to the ROR-responsive element (RORE) sites in the Ulk1a promoter, thereby repressing Ulk1a in vivo, and another autophagy-related gene, Atp6v1d, is a direct target of REV-ERBα52. All the same, information technology has also been shown that REV-ERBβ does not seem to exist directly involved in autophagy regulation but likely acts equally a cytoprotective factor downstream of a occludent of autophagy71.
Other core clock proteins also show the power to influence autophagy. AMPK activity and nuclear localization are rhythmic and are inversely correlated with Cry1 nuclear protein abundance72. The rhythmic regulator CLOCK may potentially affect tumor jail cell resistance to cisplatin by inducing autophagy73. ATG14 gene expression, which is controlled past CLOCK/BMAL1 binding to its Due east-box, also exhibits a CR74
Autophagy regulates the cyclic rhythm
Although there is relatively less show that autophagy regulates CR than that CR regulates autophagy, findings related to the core components of autophagy and CR are included in such evidence. The silencing of Tor in Per-expressing cells shortens the period of the locomotor activity rhythm of flies59. The stimulation of AMPK destabilizes cryptochromes and alters CR, and mice in which the AMPK pathway is genetically disrupted show alterations in their peripheral clocks72. The circadian proteins BMAL1, CLOCK, REV-ERBα, and Cry1 are lysosomal targets, and the selective autophagic degradation of Cry1 occurs in a diurnal window when rodents rely on gluconeogenesis, suggesting that Cry1 degradation is fourth dimension imprinted for the maintenance of blood glucose75. In addition, high-fat feeding accelerates autophagic Cry1 deposition and contributes to obesity-associated hyperglycemia75. A CLOCK mutant attenuates BMAL1 degradation through both proteasomal and autophagic pathways nether high-fat diet feeding, providing bear witness of autophagic regulation of a core clock component76.
Indirect regulation betwixt autophagy and the cyclic rhythm in intervertebral discs
The cyclic regulator CLOCK is a histone acetyltransferase (HAT) that besides acetylates a nonhistone substrate: its own partner, BMAL177. Both nonhistone acetyltransferase and HAT activities are essential to the circadian rhythmicity and activation of clock genes77. Sirt1 is a circadian deacetylase for core clock components78. The NAD(+)-dependent enzyme Sirt1, which functions as a histone deacetylase whose activeness is also regulated past the redox states of NAD cofactors, counteracts the activity of CLOCK77. Additionally, Sirt1 binds to CLOCK-BMAL1 and Per2 in a cyclic way and supports the deacetylation and degradation of Per279. In the absence of Sirt1, constitutively high protein levels of Per2 may lead to the repression of Per1, Per2, Cry1, and retinoic acid-related orphan receptor γ (RORγ) mRNA expression79. On the other paw, Sirt1 may activate autophagy through the Sirt1-LKB1-AMPK pathway and by deacetylating ATG5, ATG7, LC3, and tuberous sclerosis complex two (TSC2), a component of the mTOR inhibitory complex upstream of mTORC1fourscore,81,82,83. Taken together, these findings advise that Sirt1 serves equally a link between autophagy and CR and between the redox country and the circadian clock.
Melatonin, an endocrine hormone synthesized and secreted by the pineal gland in the brain that helps to maintain CR, significantly enhances protective effects in unlike systems, including the central nervous, cardiovascular, gastrointestinal and endocrine systems, through the enhancement or inhibition of the autophagy process84. It is believed that oxidative stress tin activate autophagy; for this reason, the antioxidant action of melatonin could account for its inhibitory effects on autophagy85. If so, melatonin may affect mechanisms that stimulate autophagy, rather than affecting the process itself85. Melatonin seems to inhibit autophagy triggered past either mTOR activation or JNK/Bcl-2/Beclin1 pathway signaling86,87. Cyclosporine A is known to induce autophagy via ER stress88. Melatonin suppresses cyclosporine-induced autophagy in rat pituitary GH3 cells through the MAPK/ERK pathway, an consequence that is due either totally or in function to the antioxidant properties of melatonin85,88. Deficiency of the nuclear melatonin receptor RORα aggravates autophagy dysfunction in diabetic hearts and myocardial ischemia/reperfusion injury in mice89,90,91. However, in regimen i-treated N2a/APP cells, just a slight increase in cellular autophagy has been plant using period cytometry, and there is no significant alteration in the expression of the autophagy-associated markers Beclin-1 and LC3-I/Ii92. These results propose that autophagy may play a negligible role in the beneficial effects of caffeine, melatonin, and coffee on Advertising92.
The circadian regulation of C/EBPβ and the autophagy disruption observed in mice lacking a functional liver clock suggest that C/EBPβ is a primal cistron that links autophagy to the biological clock and maintains food homeostasis throughout low-cal/night cycles13. In zebrafish, a CLOCK-BMAL1 heterodimer binds to E-boxes to regulate the transcription of C/EBPβ, which in turn controls the transcription of autophagy-related genes indirectlyfifty.
Fus1, a tumor suppressor protein residing in mitochondria, maintains mitochondrial homeostasis and is highly expressed in the encephalon93. I study revealed that KO mice showed sleep/wake disturbances compared to WT mice and that the autophagy mark LC3-II was decreased in both the olfactory bulbs and hippocampi, suggesting an early onset of autophagy dysregulation in Fus1 KO mice93. Heme oxygenase (Ho), whose silencing results in the downregulation of autophagy-related genes in both light and dark phases, is expressed in a cyclic mode94. FoxOs are tightly controlled by fasting/feeding cycles and tin upregulate the expression of ATG1474. Casein kinase 1α (CK1α) exhibits dual functions in autophagy regulation95. CK1α-mediated phosphorylation stimulates the degradation of Per1, suggesting a function in CR95.
The expression of the core clock genes Per2 and REV-ERBα is increased afterward weight loss96. Clock gene expression levels and their weight loss-induced changes are tightly correlated with each other and with the expression of genes involved in autophagy (LC3A and LC3B)96. Folic acid deprivation increases autophagic activity in hippocampal neuron cells, an result that is associated with the activation of autophagy- and circadian-related genes97.
In the brain, acrylamide (ACR), a chronic neurotoxin, substantially attenuates spontaneous alternation. ACR dampens the oscillatory amplitudes of clock genes (BMAL1, Cry2 and REV-ERBβ) or causes a phase shift in clock genes (CLOCK, Per2 and REV-ERBα) and weakens the amplitude of Sirt1 oscillations. In improver, ACR increases the number of autophagic structures only in the nighttime phase, which may crusade nerve cell damage and apoptosis, ultimately leading to cognitive harm (Fig. 3)98.
Discussion and prospects
To the best of our noesis, there are few studies linking autophagy and CR in IVDs. Notwithstanding, the findings reported to date provide some insights and clues that CR induces IDD at to the lowest degree partially in an autophagic style. Food condition, oxidative stress, inflammation, the osmotic environment and mechanical loading are the most common factors that induce changes in autophagy and CR in IVDs. Considering the great bargain of testify regarding nutritional status42,56,60,61,74,75,76, we are also particularly interested in this field. As nosotros described in a higher place, a periodic lack of nutrition favors the molecular CR, autophagy and their interaction, while a lack of nutritional oscillation, as observed in the context of diabetes, leads to dysfunction of autophagythreescore,61,74. Limited nutritional deprivation tin can activate autophagy and pb to cell renewal in IVDs99,100,101. Thus, we can infer that nutrition is an incentive for CR, leading to the farther regulation of autophagy. Redox status is also a prevalent gene involved in many metabolic processes. Non just HtwoO2 but besides an unsuitable nutrient status induces autophagy in an ROS-dependent manner in IVDs101. Sirt1 and melatonin are bridges between redox status and autophagy; 1 of them regulates clock-related molecules, while the other is regulated by CR77,84. It has long been known that night shift work is a pregnant adventure factor for the development of IDD and its progression102. SF has been indicated to blunt CR and damage the expression of autophagy-related proteins in the CNS, perhaps permanently51. Given all of these findings, we can enquire whether sleep rhythms regulate autophagy in IVDs. Night shift work besides involves abnormal loading/resting cycles102. In these cycles, IVDs undergo changes in the mechanical loading and activation of autophagy, which tin can consequence in IDD45. Every bit the most recent research has shown, inflammation is a pregnant gene in IDD, as IL-1β abolishes the normal molecular functions of clock-related components, participates in multiple pathological processes during disc degeneration (including inflammatory responses, matrix destruction, angiogenesis and innervation, apoptosis, oxidative stress and cellular senescence), and activates protective autophagy in IVDs103. Yet, despite this contradictory evidence, one affair is articulate: clock dysfunction is a key factor associated with inflammation-related autophagy in IDD. The water content varies in IVDs during the loading/resting cycle together with changes in the osmotic environment5,xx,22. The osmotic environment is also associated with autophagy in IVDs43. Whether this phenomenon affects the molecular CR in IVDs could be investigated in the future. Given the cadre circadian regulation observed in IVDs and the autophagy regulation mediated by components, such every bit Per65, BMAL167,68 and REV-ERB70 in other models, we can hypothesize that at that place is a fairly shut interaction betwixt CR and autophagy.
We tin can infer that express autophagy coordinated by CR protects IVDs from degeneration, while excessive autophagy and autophagic dysfunction induced past abnormal CRs under different clinical conditions accelerate IDD. Since an intrinsic CR has already been found in IVDs, farther inquiry should investigate the molecular machinery by which CR influences autophagy in the process of IDD.
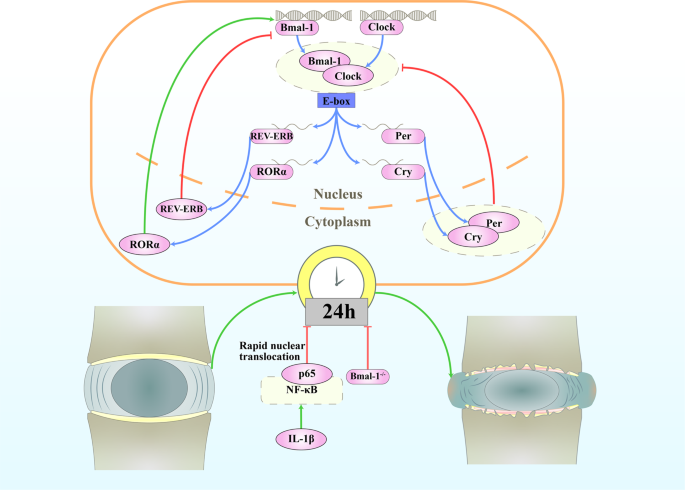
The amplitude of the oscillations in IVDs from aged mice is attenuated, with decreased expression of the core circadian transcription factors BMAL1 and CLOCK. Knockout of Bmal1 in mice IVD cells leads to the widespread degeneration of lumbar IVDs. Bone bridges appear within the growth plate, the CEP is nigh completely replaced by bone, and the top of the disc is significantly reduced. Disorganization of the outer annulus structure and signs of fibrosis (with organized collagen bundles) appear at the periphery of the IVDs. IL-1β causes the rapid nuclear translocation of p65 in both AF and NP cells and leads to a loss of pacemaking properties of individual cells.
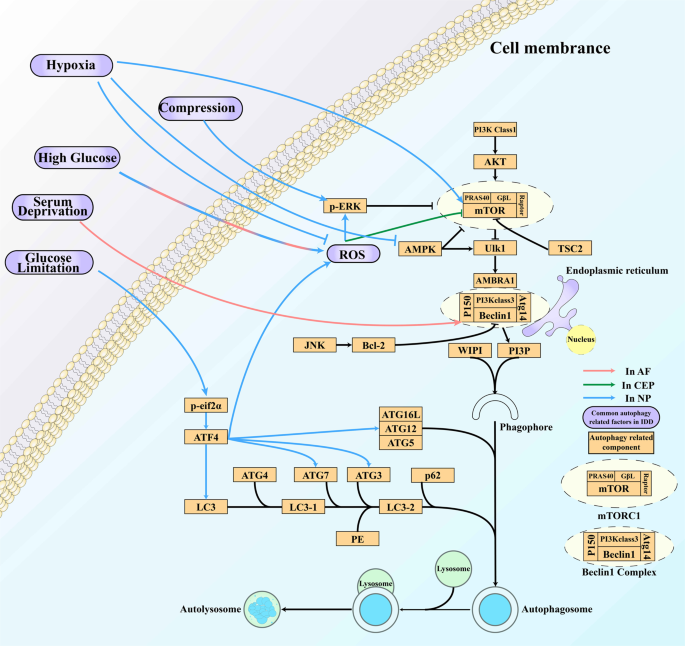
Compression, high glucose levels, serum deprivation, and glucose limitation are common stimuli that activate autophagy in IVDs through different pathways and play various roles in IDD. In contrast, hypoxia inhibits autophagy through the archetype autophagy pathway in NPCs. ROS are factors involved in high glucose-, serum deprivation- and hypoxia-dependent autophagy regulation that downregulate mTOR signaling.
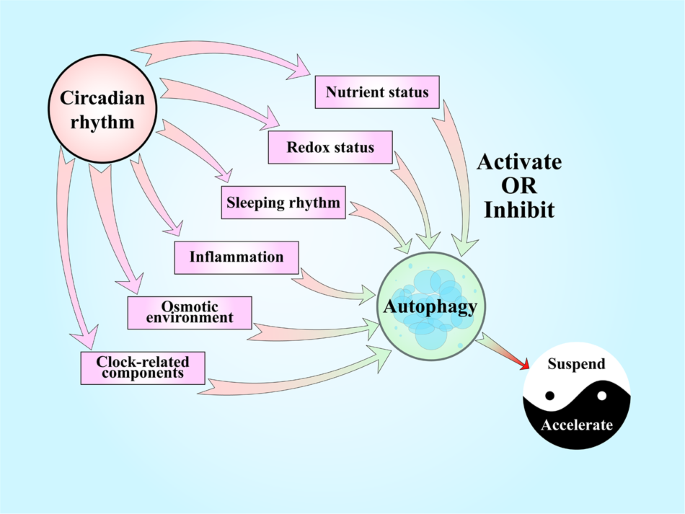
Food status, redox condition, slumber rhythms, inflammation, and the osmotic environment are common factors affecting IVDs that testify circadian rhythms in normal conditions and nowadays close connections with autophagy. The intensity and elapsing of these stimuli determine the consequences of autophagy, affecting IDD. Clock-related components accept recently been constitute to play protective roles in IVDs accept been shown to bear upon autophagy in other organs.
References
-
Deyo, R. A. & Weinstein, J. N. Depression dorsum hurting. N. Engl. J. Med 344, 363–370 (2001).
-
Pattappa, G. et al. Diversity of intervertebral disc cells: phenotype and function. J. Anat. 221, 480–496 (2012).
-
Urban, J. P., Smith, S. & Fairbank, J. C. Nutrition of the intervertebral disc. Spine (Philos. Pa 1976) 29, 2700–2709 (2004).
-
Yuan, Due west. et al. Establishment of intervertebral disc degeneration model induced by ischemic sub-endplate in rat tail. Spine J. 15, 1050–1059 (2015).
-
Massey, C. J., van Donkelaar, C. C., Vresilovic, E., Zavaliangos, A. & Marcolongo, M. Effects of aging and degeneration on the man intervertebral disc during the diurnal bike: a finite element written report. J. Orthop. Res 30, 122–128 (2012).
-
Sivan, Due south., Neidlinger-Wilke, C., Wurtz, One thousand., Maroudas, A. & Urban, J. P. Diurnal fluid expression and activity of intervertebral disc cells. Biorheology 43, 283–291 (2006).
-
Numaguchi, Southward. et al. Passive cigarette smoking changes the circadian rhythm of clock genes in rat intervertebral discs. J. Orthop. Res 34, 39–47 (2016).
-
Dudek, K. et al. The intervertebral disc contains intrinsic circadian clocks that are regulated by age and cytokines and linked to degeneration. Ann. Rheum. Dis. 76, 576–584 (2017).
-
Akagi, R. et al. Dysregulated circadian rhythm pathway in human osteoarthritis: NR1D1 and BMAL1 suppression alters TGF-beta signaling in chondrocytes. Osteoarthr. Cartil. 25, 943–951 (2017).
-
Green, C. B., Takahashi, J. S. & Bass, J. The meter of metabolism. Jail cell 134, 728–742 (2008).
-
Rubinsztein, D. C., Marino, G. & Kroemer, Grand. Autophagy and aging. Cell 146, 682–695 (2011).
-
Ito, M. et al. Selective interference of mTORC1/RAPTOR protects against homo disc cellular apoptosis, senescence, and extracellular matrix catabolism with Akt and autophagy induction. Osteoarthr. Cartil. 25, 2134–2146 (2017).
-
Ma, D., Panda, S. & Lin, J. D. Temporal orchestration of cyclic autophagy rhythm past C/EBPbeta. EMBO J. 30, 4642–4651 (2011).
-
Dibner, C., Schibler, U. & Albrecht, U. The mammalian circadian timing system: arrangement and coordination of central and peripheral clocks. Annu Rev. Physiol. 72, 517–549 (2010).
-
Partch, C. 50., Green, C. B. & Takahashi, J. Southward. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 24, 90–99 (2014).
-
Reddy, A. B. & Rey, G. Metabolic and nontranscriptional circadian clocks: eukaryotes. Annu Rev. Biochem 83, 165–189 (2014).
-
Rey, Grand. & Reddy, A. B. Connecting cellular metabolism to circadian clocks. Trends Jail cell Biol. 23, 234–241 (2013).
-
Janich, P., Meng, Q. J. & Benitah, S. A. Cyclic control of tissue homeostasis and developed stalk cells. Curr. Opin. Cell Biol. 31, 8–xv (2014).
-
Reilly, T., Tyrrell, A. & Troup, J. D. Circadian variation in man stature. Chronobiol. Int 1, 121–126 (1984).
-
Kramer, J. & Gritz, A. [Changes in torso length by pressure dependent fluid shifts in the intervertebral discs (author's transl)]. Z. Orthop. Ihre Grenzgeb. 118, 161–164 (1980).
-
Adams, 1000. A., Dolan, P. & Hutton, W. C. Diurnal variations in the stresses on the lumbar spine. Spine (Philos. Pa 1976) 12, 130–137 (1987).
-
Gantenbein, B. et al. An in vitro organ culturing organization for intervertebral disc explants with vertebral endplates: a feasibility study with ovine caudal discs. Spine (Philos. Pa 1976) 31, 2665–2673 (2006).
-
Suyama, K. et al. Circadian factors BMAL1 and RORalpha command HIF-1alpha transcriptional activity in nucleus pulposus cells: implications in maintenance of intervertebral disc health. Oncotarget 7, 23056–23071 (2016).
-
Wu, D., Potluri, N., Lu, J., Kim, Y. & Rastinejad, F. Structural integration in hypoxia-inducible factors. Nature 524, 303–308 (2015).
-
Codogno, P., Mehrpour, M. & Proikas-Cezanne, T. Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nat. Rev. Mol. Cell Biol. xiii, vii–12 (2011).
-
Kim, J., Kundu, M., Viollet, B. & Guan, One thousand. L. AMPK and mTOR regulate autophagy through straight phosphorylation of Ulk1. Nat. Prison cell Biol. thirteen, 132–141 (2011).
-
Hosokawa, Due north. et al. Food-dependent mTORC1 association with the ULK1-Atg13-FIP200 circuitous required for autophagy. Mol. Biol. Jail cell 20, 1981–1991 (2009).
-
Lee, J. W., Park, Due south., Takahashi, Y. & Wang, H. G. The clan of AMPK with ULK1 regulates autophagy. PLoS ONE 5, e15394 (2010).
-
Grimmel, Grand., Backhaus, C. & Proikas-Cezanne, T. WIPI-Mediated Autophagy and Longevity. Cells 4, 202–217 (2015).
-
Di Bartolomeo, S. et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J. Jail cell Biol. 191, 155–168 (2010).
-
Itakura, E. & Mizushima, Northward. Atg14 and UVRAG: mutually sectional subunits of mammalian Beclin i-PI3K complexes. Autophagy 5, 534–536 (2009).
-
Mizushima, N. & Komatsu, M. Autophagy: renovation of cells and tissues. Cell 147, 728–741 (2011).
-
Yang, Z. & Klionsky, D. J. Mammalian autophagy: core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 22, 124–131 (2010).
-
Levine, B. & Kroemer, 1000. Autophagy in the pathogenesis of disease. Cell 132, 27–42 (2008).
-
Xu, H., Xiong, Due south., Wang, H., Zhang, M. & Yu, Y. The evidence and the possible significance of autophagy in degeneration model of man cervical end-plate cartilage. Exp. Ther. Med 7, 537–542 (2014).
-
Yu, Y. F. et al. [Change of autophagy in endplate chondrocytes of rats during aging process]. Zhonghua Yi Xue Za Zhi 93, 3632–3635 (2013).
-
Ye, W. et al. Age-related increases of macroautophagy and chaperone-mediated autophagy in rat nucleus pulposus. Connect Tissue Res 52, 472–478 (2011).
-
Gruber, H. East., Hoelscher, G. L., Ingram, J. A., Bethea, S. & Hanley, E. North. Jr. Autophagy in the degenerating human being intervertebral disc: in vivo molecular and morphological evidence, and consecration of autophagy in cultured annulus cells exposed to proinflammatory cytokines-implications for disc degeneration. Spine (Philos. Pa 1976) forty, 773–782 (2015).
-
Shen, J. et al. IL-1beta induces apoptosis and autophagy via mitochondria pathway in human degenerative nucleus pulposus cells. Sci. Rep. vii, 41067 (2017).
-
Chen, L. et al. Protein kinase RNA-like ER kinase/eukaryotic translation initiation gene 2alpha pathway attenuates tumor necrosis cistron alpha-induced apoptosis in nucleus pulposus cells by activating autophagy. J. Cell Physiol. 234, 11631–11645 (2019).
-
Xu, Thousand. et al. Autophagy attenuates the catabolic event during inflammatory conditions in nucleus pulposus cells, as sustained past NF-kappaB and JNK inhibition. Int J. Mol. Med 36, 661–668 (2015).
-
Shen, C., Yan, J., Jiang, 50. S. & Dai, L. Y. Autophagy in rat annulus fibrosus cells: evidence and possible implications. Arthritis Res Ther. 13, R132 (2011).
-
Jiang, L. B. et al. Activation of autophagy via Ca(2+)-dependent AMPK/mTOR pathway in rat notochordal cells is a cellular adaptation nether hyperosmotic stress. Cell Bicycle 14, 867–879 (2015).
-
Liu, C. et al. Lack of evidence for involvement of TonEBP and hyperosmotic stimulus in induction of autophagy in the nucleus pulposus. Sci. Rep. 7, 4543 (2017).
-
Xu, H. G. et al. Autophagy protects terminate plate chondrocytes from intermittent cyclic mechanical tension induced calcification. Os 66, 232–239 (2014).
-
Kakiuchi, Y. et al. Pharmacological inhibition of mTORC1 but not mTORC2 protects against homo disc cellular apoptosis, senescence, and extracellular matrix catabolism through Akt and autophagy consecration. Osteoarthr. Cartil. 27, 965–976 (2019).
-
Pfeifer, U. Cellular autophagy and jail cell atrophy in the rat liver during long-term starvation. A quantitative morphological study with regard to diurnal variations. Virchows Arch. B Prison cell Pathol. 12, 195–211 (1973).
-
Pfeifer, U. & Strauss, P. Autophagic vacuoles in heart musculus and liver. A comparative morphometric study including circadian variations in meal-fed rats. J. Mol. Cell Cardiol. thirteen, 37–49 (1981).
-
Yao, J. et al. Circadian and noncircadian modulation of autophagy in photoreceptors and retinal pigment epithelium. Invest Ophthalmol. Vis. Sci. 55, 3237–3246 (2014).
-
Huang, G., Zhang, F., Ye, Q. & Wang, H. The cyclic clock regulates autophagy straight through the nuclear hormone receptor Nr1d1/Rev-erbalpha and indirectly via Cebpb/(C/ebpbeta) in zebrafish. Autophagy 12, 1292–1309 (2016).
-
He, Y. et al. Circadian rhythm of autophagy proteins in hippocampus is blunted past sleep fragmentation. Chronobiol. Int 33, 553–560 (2016).
-
Reme, C. & Wirz-Justice, A. [Circadian rhythm, the retina and lite]. Klin. Monbl Augenheilkd. 186, 175–179 (1985).
-
Rabinovich-Nikitin, I., Lieberman, B., Martino, T. A. & Kirshenbaum, L. A. Circadian-regulated cell decease in cardiovascular diseases. Circulation 139, 965–980 (2019).
-
Reme, C., Wirzjustice, A., Rhyner, A. & Hofmann, Southward. Circadian-rhythm in the low-cal response of rat retinal disk-shedding and autophagy. Brain Res. 369, 356–360 (1986).
-
Frost, L. South. et al. The contribution of melanoregulin to microtubule-associated protein 1 calorie-free chain 3 (LC3) associated phagocytosis in retinal pigment epithelium. Mol. Neurobiol. 52, 1135–1151 (2015).
-
Ryzhikov, Grand. et al. Diurnal rhythms spatially and temporally organize autophagy. Jail cell Rep. 26, 1880–1892 e1886 (2019).
-
Chen, X., Kondo, One thousand., Motoki, G., Homma, H. & Okazawa, H. Fasting activates macroautophagy in neurons of Alzheimer'southward affliction mouse model but is insufficient to dethrone amyloid-beta. Sci. Rep. five, 12115 (2015).
-
Pfeifer, U. & Scheller, H. A morphometric study of cellular autophagy including diurnal variations in kidney tubules of normal rats. J. Prison cell Biol. 64, 608–621 (1975).
-
Kijak, Due east. & Pyza, East. TOR signaling pathway and autophagy are involved in the regulation of circadian rhythms in behavior and plasticity of L2 interneurons in the brain of Drosophila melanogaster. PLoS I 12, e0171848 (2017).
-
Martinez-Lopez, N. et al. System-broad benefits of intermeal fasting by autophagy. Cell Metab. 26, 856–871 e855 (2017).
-
Stockman, M. C., Thomas, D., Burke, J. & Apovian, C. M. Intermittent fasting: is the wait worth the weight? Curr. Obes. Rep. vii, 172–185 (2018).
-
Reme, C., Wirz-Justice, A., Rhyner, A. & Hofmann, S. Cyclic rhythm in the low-cal response of rat retinal disk-shedding and autophagy. Brain Res 369, 356–360 (1986).
-
Mohlin, C., Taylor, 50., Ghosh, F. & Johansson, One thousand. Autophagy and ER-stress contribute to photoreceptor degeneration in cultured adult porcine retina. Brain Res 1585, 167–183 (2014).
-
Wu, R. et al. The cyclic protein period2 suppresses mTORC1 activity via recruiting Tsc1 to mTORC1 complex. Jail cell Metab. 29, 653–667 e656 (2019).
-
Kalfalah, F. et al. Crosstalk of clock gene expression and autophagy in aging. Aging (Albany NY) eight, 1876–1895 (2016).
-
Wang, Z., Li, Fifty. & Wang, Y. Effects of Per2 overexpression on growth inhibition and metastasis, and on MTA1, nm23-H1 and the autophagy-associated PI3K/PKB signaling pathway in nude mice xenograft models of ovarian cancer. Mol. Med Rep. 13, 4561–4568 (2016).
-
Qiao, L. et al. The clock cistron, brain and muscle Arnt-like ane, regulates autophagy in high glucose-induced cardiomyocyte injury. Oncotarget 8, 80612–80624 (2017).
-
Scotton, C. et al. Deep RNA profiling identified CLOCK and molecular clock genes as pathophysiological signatures in collagen VI myopathy. J. Cell Sci. 129, 1671–1684 (2016).
-
McGinnis, G. R. et al. Genetic disruption of the cardiomyocyte circadian clock differentially influences insulin-mediated processes in the center. J. Mol. Prison cell Cardiol. 110, 80–95 (2017).
-
Sulli, G. et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 553, 351–355 (2018).
-
De Mei, C. et al. Dual inhibition of REV-ERBbeta and autophagy as a novel pharmacological approach to induce cytotoxicity in cancer cells. Oncogene 34, 2597–2608 (2015).
-
Lamia, G. A. et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326, 437–440 (2009).
-
Sun, Y., Jin, L., Sui, Y. X., Han, L. L. & Liu, J. H. Circadian gene CLOCK Affects drug-resistant gene expression and prison cell proliferation in ovarian cancer SKOV3/DDP Jail cell lines through autophagy. Cancer Biother Radiopharm. 32, 139–146 (2017).
-
Xiong, X., Tao, R., DePinho, R. A. & Dong, X. C. The autophagy-related factor 14 (Atg14) is regulated by forkhead box O transcription factors and cyclic rhythms and plays a critical role in hepatic autophagy and lipid metabolism. J. Biol. Chem. 287, 39107–39114 (2012).
-
Toledo, M. et al. Autophagy regulates the liver clock and glucose metabolism past degrading CRY1. Cell Metab. 28, 268–281 e264 (2018).
-
Jeong, K. et al. Dual attenuation of proteasomal and autophagic BMAL1 deposition in Clock Delta19/+ mice contributes to improved glucose homeostasis. Sci. Rep. 5, 12801 (2015).
-
Nakahata, Y. et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and cyclic control. Cell 134, 329–340 (2008).
-
Belden, W. J. & Dunlap, J. C. SIRT1 is a circadian deacetylase for core clock components. Cell 134, 212–214 (2008).
-
Asher, K. et al. SIRT1 regulates cyclic clock gene expression through PER2 deacetylation. Cell 134, 317–328 (2008).
-
Ghosh, H. S., McBurney, M. & Robbins, P. D. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS ONE 5, e9199 (2010).
-
Lan, F., Cacicedo, J. M., Ruderman, N. & Ido, Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible part in AMP-activated poly peptide kinase activation. J. Biol. Chem. 283, 27628–27635 (2008).
-
Lee, I. H. et al. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl Acad. Sci. USA 105, 3374–3379 (2008).
-
Chung, S. et al. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch. Biochem Biophys. 501, 79–90 (2010).
-
Roohbakhsh, A., Shamsizadeh, A., Hayes, A. Westward., Reiter, R. J. & Karimi, Thousand. Melatonin every bit an endogenous regulator of diseases: the role of autophagy. Pharmacol. Res. 133, 265–276 (2018).
-
Motilva, 5., Garcia-Maurino, S., Talero, Eastward. & Illanes, M. New paradigms in chronic intestinal inflammation and colon cancer: part of melatonin. J. Pineal Res 51, 44–sixty (2011).
-
Kongsuphol, P., Mukda, Southward., Nopparat, C., Villarroel, A. & Govitrapong, P. Melatonin attenuates methamphetamine-induced deactivation of the mammalian target of rapamycin signaling to induce autophagy in SK-North-SH cells. J. Pineal Res 46, 199–206 (2009).
-
Nopparat, C., Porter, J. E., Ebadi, M. & Govitrapong, P. The mechanism for the neuroprotective upshot of melatonin against methamphetamine-induced autophagy. J. Pineal Res 49, 382–389 (2010).
-
Yoo, Y. K. & Jeung, Eastward. B. Melatonin suppresses cyclosporine A-induced autophagy in rat pituitary GH3 cells. J. Pineal Res 48, 204–211 (2010).
-
Jenwitheesuk, A., Nopparat, C., Mukda, S., Wongchitrat, P. & Govitrapong, P. Melatonin regulates aging and neurodegeneration through energy metabolism, epigenetics, autophagy and circadian rhythm pathways. Int J. Mol. Sci. 15, 16848–16884 (2014).
-
Zhao, Y. et al. Novel protective part of the circadian nuclear receptor retinoic acid-related orphan receptor-alpha in diabetic cardiomyopathy. J Pineal Res 62, https://doi.org/ten.1111/jpi.12378 (2017).
-
He, B. et al. The nuclear melatonin receptor RORalpha is a novel endogenous defender against myocardial ischemia/reperfusion injury. J. Pineal Res 60, 313–326 (2016).
-
Zhang, L. F. et al. Coffee and caffeine potentiate the antiamyloidogenic activity of melatonin via inhibition of Abeta oligomerization and modulation of the Tau-mediated pathway in N2a/APP cells. Drug Des. Devel Ther. nine, 241–272 (2015).
-
Coronas-Samano, Thousand., Baker, K. 50., Tan, West. J., Ivanova, A. 5. & Verhagen, J. 5. Fus1 KO mouse as a model of oxidative stress-mediated sporadic Alzheimer's affliction: circadian disruption and long-term spatial and olfactory memory impairments. Front Aging Neurosci. 8, 268 (2016).
-
Damulewicz, Chiliad. et al. Daily regulation of phototransduction, circadian clock, Deoxyribonucleic acid repair, and immune gene expression by heme oxygenase in the retina of Drosophila. Genes (Basel) 10, six (2018).
-
Jiang, Due south., Zhang, 1000., Sun, J. & Yang, X. Casein kinase 1alpha: biological mechanisms and theranostic potential. Cell Commun. Signal 16, 23 (2018).
-
Pivovarova, O. et al. Regulation of the clock gene expression in human adipose tissue by weight loss. Int J. Obes. (Lond.) 40, 899–906 (2016).
-
Lord's day, Q. et al. Folate deprivation modulates the expression of autophagy- and circadian-related genes in HT-22 hippocampal neuron cells through GR-mediated pathway. Steroids 112, 12–19 (2016).
-
Tan, X. et al. Acrylamide aggravates cognitive deficits at nighttime period via the gut-brain axis by reprogramming the brain circadian clock. Arch. Toxicol. 93, 467–486 (2019).
-
Bretin, A. et al. Activation of the EIF2AK4-EIF2A/eIF2alpha-ATF4 pathway triggers autophagy response to Crohn disease-associated adherent-invasive Escherichia coli infection. Autophagy 12, 770–783 (2016).
-
Chang, H. et al. Early-stage autophagy protects nucleus pulposus cells from glucose impecuniousness-induced degeneration via the p-eIF2alpha/ATF4 pathway. Biomed. Pharmacother. 89, 529–535 (2017).
-
Chen, J. Due west. et al. Hypoxia facilitates the survival of nucleus pulposus cells in serum impecuniousness by down-regulating excessive autophagy through restricting ROS generation. Int J. Biochem Cell Biol. 59, one–10 (2015).
-
Elfering, A. et al. Risk factors for lumbar disc degeneration: a v-yr prospective MRI study in asymptomatic individuals. Spine (Philos. Pa 1976) 27, 125–134 (2002).
-
Yang, Due west. et al. Interleukin-1beta in intervertebral disk degeneration. Clin. Chim. Acta 450, 262–272 (2015).
Acknowledgements
This work was supported by the National Natural Scientific discipline Foundation of People's republic of china (no. 81702177; no. 81772855; no. 81572629) and the Shanghai Sailing Programme (17YF1402300).
Author data
Affiliations
Corresponding authors
Ethics declarations
Conflict of involvement
The authors declare that they have no conflict of involvement.
Additional information
Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open up Access This article is licensed under a Creative Eatables Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, equally long every bit you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the commodity'due south Artistic Eatables license, unless indicated otherwise in a credit line to the material. If material is not included in the commodity'southward Creative Commons license and your intended use is non permitted by statutory regulation or exceeds the permitted use, yous will need to obtain permission straight from the copyright holder. To view a re-create of this license, visit http://creativecommons.org/licenses/by/four.0/.
Reprints and Permissions
About this article
Cite this commodity
Zhang, TW., Li, ZF., Dong, J. et al. The circadian rhythm in intervertebral disc degeneration: an autophagy connection. Exp Mol Med 52, 31–40 (2020). https://doi.org/10.1038/s12276-019-0372-6
-
Received:
-
Revised:
-
Accustomed:
-
Published:
-
Issue Appointment:
-
DOI : https://doi.org/10.1038/s12276-019-0372-half dozen
Further reading
Source: https://www.nature.com/articles/s12276-019-0372-6
Posted by: christianhaterequed.blogspot.com


0 Response to "Can Autophagy Help Repair Back Discs"
Post a Comment